STAIR XII meeting in Washington DC
Over 20 years ago, Marc Fisher, Professor of Neurology at Harvard Medical School and editor-in-chief of “Stroke”, launched the conference called STAIR (Stroke Treatment Academic Industry Roundtable). The aim was to advance and accelerate stroke research to improve acute stroke treatment, care, and prevention by bringing together medical academia, industry leaders, and U.S. FDA regulators. As an industry partner whose research activities are in line with these goals, EVER Pharma was also represented at this year's STAIR XII meeting in Washington DC.

Why was EVER Pharma invited?
Currently, 14 academical centers worldwide are participating in a program called CERECAP (Cerebrolysin RECanalization And Perfusion). These research groups have developed clinical study projects to investigate the role of Cerebrolysin® as add-on therapy in recanalization treatments after acute ischemic stroke (AIS). With the invitation of EVER Pharma by the STAIR organizing team, the appreciation of this program and the first successes that have already been published were taken into account.
In fact, many lectures and panel discussions within the framework of the STAIR conference agenda are relevant for the CERECAP program, such as:
- trials with cytoprotective agents prior to reperfusion
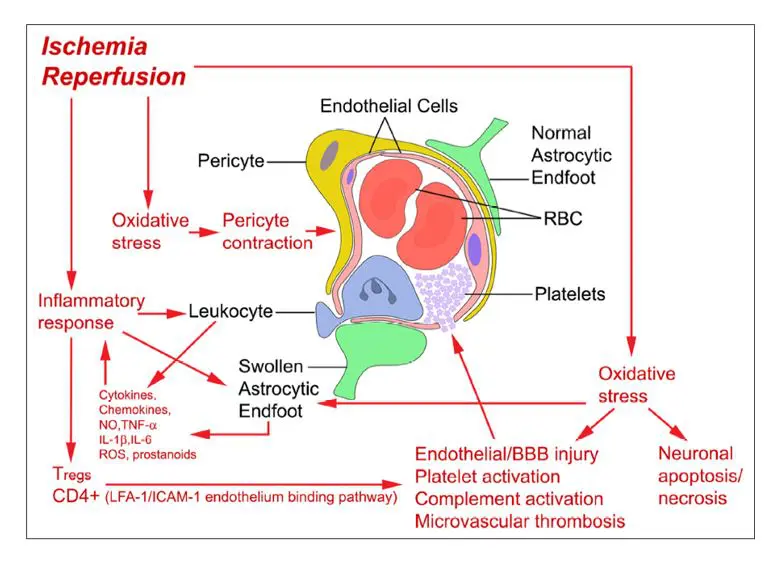
- treatments for reperfusion injuries
- identification of factors that lead to poor outcomes despite successful reperfusion
- improving the outcome of recanalization treatment
The various studies within the framework of the CERECAP program deal with these problems and the publications to date have shown that the administration of Cerebrolysin®
- is safe when given in ambulances and mobile stroke units (CERECAP – Thailand)
- reduces the occurrence of all forms of hemorrhagic transformation (CERECAP – Croatia)
- improves the BBB integrity (CERECAP – USA; preclinical)
Participation in STAIR – Opportunities for EVER Pharma
At the STAIR conference, delegates also participated in interactive workshops aimed at developing consensus-based recommendations, which will be published in “Stroke” in the 3rd quarter of 2023.
At the workshop entitled “Most promising approaches to improve stroke outcomes”, Ever Pharma was able to communicate the ongoing research projects and highlight that the new terminology proposed by Prof. Lyden - cerebroprotection or brain cytoprotection – actually expresses more precisely that effective agents must influence processes of the neurovascular unit. This can only be achieved with multimodal agents such as Cerebrolysin®. This is also in line with another consensus statement by STAIR XI:
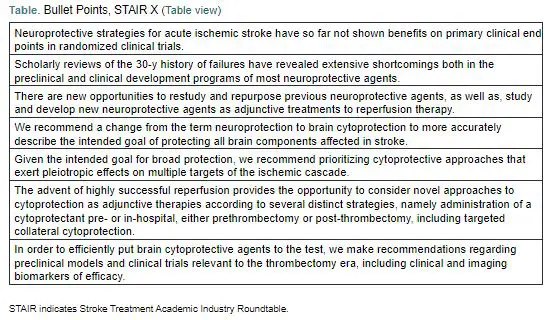
"We recommend prioritizing cytoprotective approaches that exert pleiotropic effects on multiple targets of the ischemic cascade."
Summary
With CERECAP, EVER Pharma has developed a research program that attracts the attention of the global KOLs in stroke medicine. EVER Pharma has gladly accepted the invitation to the most prestigious forum driving stroke treatment standards. Pharmacologically, Cerebrolysin® meets the STAIR criteria almost perfectly and therefore EVER Pharma feels encouraged to continue this path of clinical development and to stimulate global research activities on the use of Cerebrolysin® in acute ischemic stroke.