CREGS study marks a major milestone in stroke research
We are proud to announce the results of the CREGS (Cerebrolysin Registry Study in Stroke) study, the largest clinical investigation ever conducted on Cerebrolysin®, involving more than 1,800 patients with acute ischemic stroke.

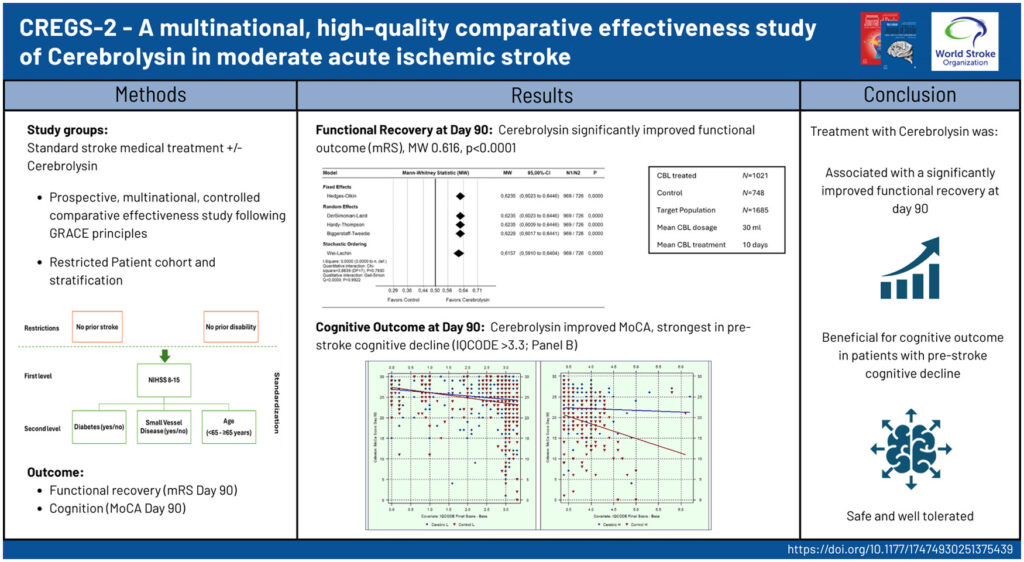
Published in the International Journal of Stroke, the study was presented today by Professor Michael Brainin in the Main Session of the World Stroke Congress — underscoring the global importance of these findings.
Outstanding Clinical Results
In the CREGS study, patients treated with Cerebrolysin® showed a significant improvement in functional recovery compared with the control group, as measured by the modified Rankin Scale (mRS) and the NIH Stroke Scale (NIHSS).
Importantly, patients who could not receive thrombolysis showed significant improvement with Cerebrolysin® treatment — offering a meaningful therapeutic option.
But also patients receiving thrombolysis benefited from significantly enhanced recovery outcomes when Cerebrolysin® was added.
Preserves Cognitive Functions
Beyond functional recovery, the study also assessed cognitive outcomes. Particularly in patients with more pronounced pre-stroke cognition problems, Cerebrolysin® helped to preserve cognitive functions, reducing the risk of cognitive decline during recovery.
Real-World Impact
The CREGS study stands out as a prospective high-quality comparative effectiveness trial, designed to evaluate how Cerebrolysin® supports patient's longterm outcome in real-world clinical settings.
These results reconfirm results from earlier randomized clnical trials that Cerebrolysin® should be administered as early as possible — ideally during the hyperacute phase — to achieve the best possible functional and cognitive outcomes.
Cerebrolysin® - First and only pharmacological agent evidence-based for all acute ischemic stroke patients
- Early and sustained functional recovery
- Effective as stand-alone therapy
- Preserves cognitive functions
- Enhances the effects of recanalization therapy
Confirmed in a global real-world data study involving 1.865 patients