About Cerebrolysin®
Safety
Cerebrolysin® is safe and well tolerated!
According to EMA classification (European Medicines Agency), Cerebrolysin® is in the SAFE category. In general, reported adverse drug reactions are transient and mild in intensity.
Please note that the handling is very important.
You can get more information in the Dosage Card.
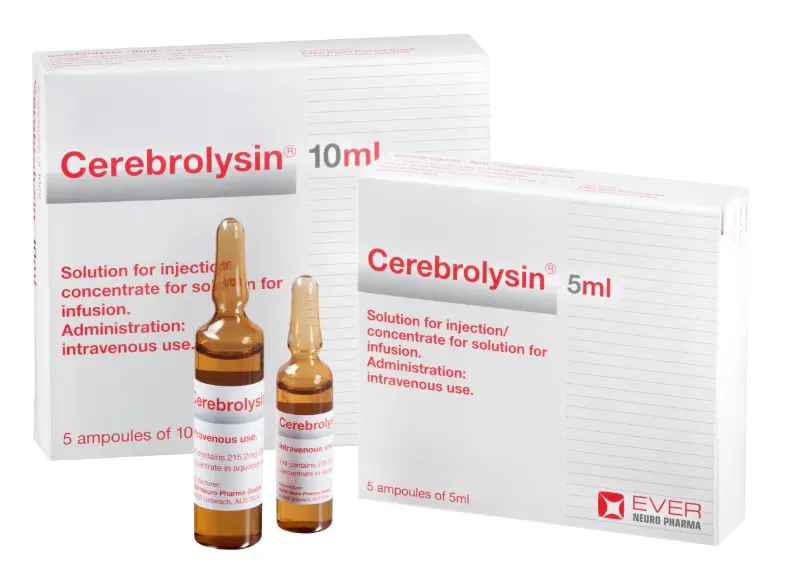
Prescribing information
Only available on prescription and in pharmacies
More information about pharmaceutical form, posology and method of administration, special warnings and precautions for use, interaction with other medicinal products and other forms of interaction, fertility, pregnancy and lactation, effects on ability to drive and use machines, undesirable effects, overdose, pharmacodynamics properties, pharmacokinetic properties, preclinical safety data, incompatibilities, shelf life, special precautions for storage, nature and contents of the container and special precautions for disposal is available in the summary of product characteristics.
This information is based on the Austrian Summary of Product Characteristics. The prescribing information in your country may vary; please consult your local prescribing information and/or contact your local EVER representative.